Counterfeit medicine has become a serious threat to patient safety. Everyday thousands of people die or suffer as a result of using counterfeit medicines. Commensurate rise in pharmaceutical counterfeiting is caused to increase the death rate potentially around the globe. World Health Organisation (WHO) says more than 120,000 people a year die in Africa as a result of fake anti-malarial drugs alone. In Singapore, four people died and seven suffered brain damage after taking counterfeit drugs to treat erectile dysfunction. It is said that 10 percent of world’s drug supply is counterfeit.
Counterfeit medicine is fake medicine that has been deliberately and fraudulently produced and mislabelled with respect to identity and source to make it appear to be genuine products. Any counterfeit pharmaceutical products might contain the right active ingredients which are of bad quality or in the wrong dose - either too high or too low. Some of the ingredients found in the counterfeit medicines included brick dust, paint, floor wax and pesticides. These products are mostly being supplied to the market proportionately to the genuine products with similar labels and logos. World Health Organisation (WHO) prefers to use it as ‘substandard, spurious, falsely-labelled, falsified and counterfeit (SSFFC) medicines. Counterfeit pharmaceutical products are often produced in very poor and unhygienic conditions.
Growth of counterfeit medicines
 Counterfeit medical products are manufactured in many different countries and in all regions. A number of factors have contributed to rise in pharmaceutical counterfeiting. Included among them are the growing involvement in the drug supply chain of under –regulated wholesalers and re-packagers, the proliferation of internet pharmacies, advancement in technology that make it easier for criminals to make falsified medicines. In a statement in 2011, the World Health Organisation cited, increasing international trade of pharmaceuticals and sales via the internet has further facilitated the entry of counterfeiters. The record shows an enormous growth in manufacturing pharmaceutical products in less developed countries over recent years. China, India and Hong Kong are among the biggest manufacturers in pharmaceutical products with 68%, 28% and 1.5% respectively.
Counterfeit medical products are manufactured in many different countries and in all regions. A number of factors have contributed to rise in pharmaceutical counterfeiting. Included among them are the growing involvement in the drug supply chain of under –regulated wholesalers and re-packagers, the proliferation of internet pharmacies, advancement in technology that make it easier for criminals to make falsified medicines. In a statement in 2011, the World Health Organisation cited, increasing international trade of pharmaceuticals and sales via the internet has further facilitated the entry of counterfeiters. The record shows an enormous growth in manufacturing pharmaceutical products in less developed countries over recent years. China, India and Hong Kong are among the biggest manufacturers in pharmaceutical products with 68%, 28% and 1.5% respectively.
The European Commission Taxation and Customs Union says, these countries being predominantly the greatest exporters to the countries in the European Union. Paul Newton, a professor of tropical medicine and director of the clinical tropical medicine research group, the Lao - Oxford – Mahosot Hospital – Wellcome Trust Research Unit, based in Laos, says: the number of falsified medicines have risen in less developed countries due to lack of regulation for counterfeiters. “However occurrences of falsified products in Europe, Australia and the United States, are relatively low when comparing with developed countries” Paul Newton describes.
During the past decades counterfeit medicine has become a huge global issue. Because appropriate systems to identify falsified pharmaceutical products are not in place especially in less developed countries. It is easier for counterfeiters to market substandard medical products in less developed countries. According to the World Health Organisation (WHO), 10 percent of world’s drug supply is counterfeit. In 2005, the sales of counterfeit drugs amount to 39 billion US Dollars and in 2010 it was estimated at 75 billion US Dollars. It is an increase of 90 percent in five years. And 60 percent of counterfeit drug supply cases originated in less developed countries.
Impact of counterfeit medicines
Counterfeit medicines can cause serious health hazardous. For instance, in the Democratic Republic of Congo, the patients were found with unusual symptoms such as protruding tongue, extended neck, facial cramps and contorted upper body after taking fake Diazepam to treat a wide range of illnesses, including malaria.
Diazepam is usually used to treat anxiety disorders, alcohol withdrawal and muscle spasms. Occasionally, doctors use it to try to control convulsions in people with malaria, if there is no alternative. The most affected people with counterfeit drugs are the poor living in developing countries. The shortage of medicines and poor access to health facilities in less developed countries causing to buy falsified or substandard medicines. For instance, the records show a higher death rate in African countries as a result of using falsified medicines.
Mick Deats, group leader at the World Health Organisation (WHO) on substandard and falsified medical products, says “the clandestine nature of counterfeit medicine manufacture makes it difficult both to track down the perpetrators and quantity the extend of the problem”.
Seizure of counterfeit medicines
UK’s Medicines and Healthcare product Regulatory Agency (MHRA) seized 15.8 million Sterling Pounds worth counterfeit and unlicensed medicines and devices on May 18, 2015 as part of a global operation. It announced that it was the biggest recorded seizure to date in the UK. It includes huge quantities of illegally supplied and potentially harmful slimming pills, erectile dysfunction tablets, anaemia tablets and narcolepsy tablets. The UK operation also resulted in 1380 websites being closed down and 339 of which were UK websites selling unlicensed or counterfeit pills and tablets. The majority of the products seized in the UK originated from India, Hong Kong and Singapore. The MHRA said criminals were making money at the expense of people’s health and it was a growing problem. The seizures were part of Operation Pangea – an international clampdown on the illegal trade in fake medicines by 115 countries.
Sri Lanka customs destroyed 17 million Sri Lankan rupees worth contraband which included 68, 400 tablets of ‘Rivotril’ imported from Pakistan and India.
In June 2013, two large boxes containing 150,000 packs of Postinor-2 were discovered at Lagos international airport by the Nigerian drug regulator. The product, a widely used emergency contraceptive containing levonorgestrel, contained no active ingredient. Similar batches have been detected in Ghana, Kenya and Angola.
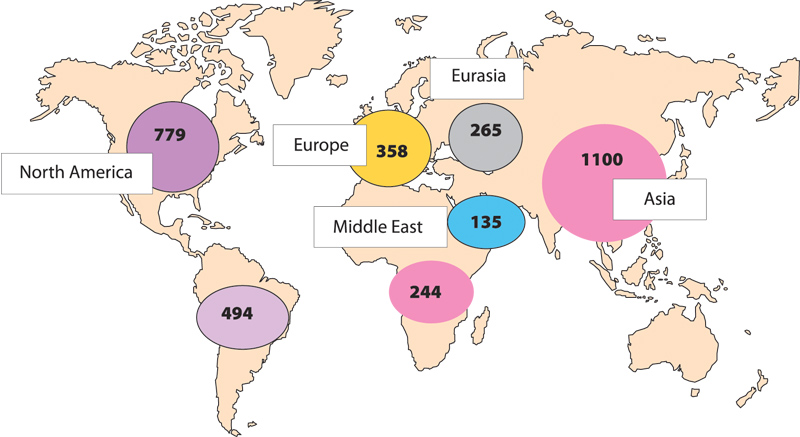
But figures from the Pharmaceutical Security Industry (PSI), a US – based not-for-profit membership organisation dedicated to protecting public health, sharing information on counterfeit drugs and initiating enforcement action, suggest that no country is immune to counterfeiting. In 2015 the PSI found that while 1,100 incidents were reported in Asia, there were 779 in North America and 358 in Europe. An incidents means that a country has been identified as the origin, point of seizure or transit, or destination of illegal pharmaceuticals.
Some frequently cited statistics include the worldwide value of counterfeit medicines being around 75 billion US Dollars. According to the US Centre for Medicine in the Public Interest and a World Economic Forum estimate in 2011 that the impact on drugs manufacturers was around 200 billion US Dollars.
More recently, the European Union Intellectual Property office estimated that the EU pharmaceutical sector loses 10.2 billion Euros a year, or 4.4 per cent of sales, to counterfeit medicine. But intellectual property law expert Iain Connor of Pinsent Masons says: “The value is at least 10, if not 100, times bigger than the reported figure”. It proves how genuine legitimate pharmaceutical manufacturers are affected due to counterfeit medical products. Professor Paul Newton says, “regulation has to improve to protect the reputations of legitimate pharmaceutical products manufacturers of both innovative and generic products”.
Risk of online purchase
Counterfeiters use online marketing to sell their products at lower price mainly targeting less developed countries because, patients may be driven to purchase medicines online due to their affordability on the prices of medicine. Alastair Jeffry, the head of enforcement of Medicines and Healthcare Regulatory Agency (MHRA) says “it is amazing to me that people will buy those types of medicines over the internet”.
Technologies for patient safety
Many pharmaceutical companies implemented technologies for patient safety. For instance, Pfizer is the first pharmaceutical company to put in place this type of comprehensive programme focusing on EPC authentication as a mean deterring counterfeiting. Pfizer also uses radio frequency identification (RFID) tags on certain highly counterfeited products in order to further ensure patient safety in the United States. RFID technology enables pharmacies and wholesalers to track medicines from manufacturer to pharmacy by verifying the unique electronic product code (EPC) on the product packaging.
The governing body of Sri Lanka should take necessary arrangements to prevent the nation from counterfeiters by implementing regulations not only protect public health, but also to protect the reputation of legitimate manufacturers.

Add new comment