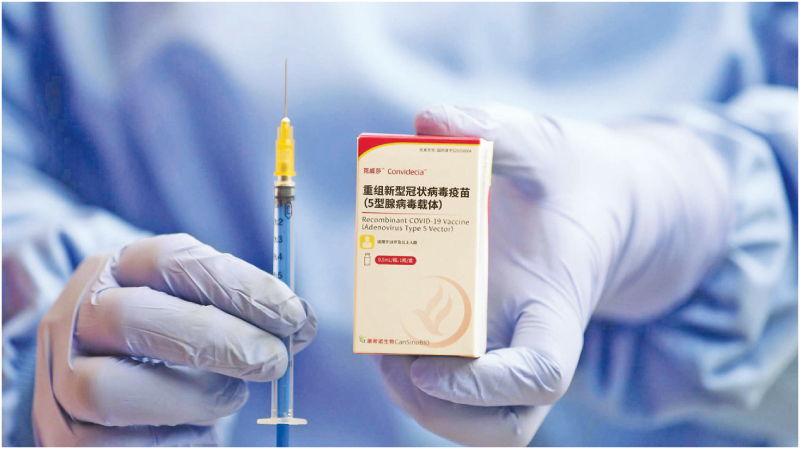
KOREA,SWITZERLAND: The World Health Organization on Thursday gave the green light to Chinese manufacturer CanSinoBIO’s COVID-19 vaccine — the ninth jab to get the WHO seal of approval.
The WHO granted emergency use listing (EUL) authorisation to the single-shot Convidecia vaccine as China battles a resurgence of the virus triggered by the Omicron variant.
It is the third Chinese-made vaccine to be approved by the UN’s health agency, after Sinovac and Sinopharm.
Convidecia was found to have 64 per cent efficacy against symptomatic disease and 92 percent efficacy against severe COVID-19, the WHO said.
“The vaccine meets WHO standards for protection against COVID-19 and ... the benefits of the vaccine far outweigh risks,” the UN health agency said in a statement.
The WHO has now given EUL status to nine COVID-19 vaccines and variations thereof — Pfizer/BioNTech, AstraZeneca, Janssen, Moderna, Sinovac, Sinopharm, Bharat Biotech, Novavax and CanSinoBIO.
Meanwhile, North Korea on Thursday reported 262,270 more suspected COVID-19 cases as its pandemic caseload neared 2 million, a week after the country acknowledged the outbreak and scrambled to slow infections in its unvaccinated population.
- THE MALAY MAIL, INDIAN EXPRESS

Add new comment